When There Be A Covid Vaccine
there wallpaperThe Moderna vaccine was approved for use on 8 th January 2021 You can access the Moderna Patient Information Leaflet here The Information for Healthcare Professionals is available here. All vaccines undergo rigorous testing and have oversight from experienced regulators.

Why We Might Not Get A Coronavirus Vaccine Coronavirus The Guardian
Linial said she believes that the reason no mRNA vaccine has been developed yet is because there was just no need to move this fast on a vaccine until COVID-19 came along.

When there be a covid vaccine. 13 2020 -- Two vaccines have been approved for use in the United States a third and fourth are. There are many potential COVID-19 vaccine candidates currently in development. Four COVID Vaccines Compared By WebMD News Staff This article was last updated Jan.
More centres are opening all the time. It now means that mass vaccination against COVID-19 could take place within days. A 45-year-old healthcare worker with a health condition would expect to get their vaccine between December 20 of this year and January 16 next year the website says.
The first doses of. The UK government is aiming to offer 15 million vaccinations by mid-February. There are of course many other vaccines being worked on and picturing the quest for a Covid vaccine as a race may be misleading.
Participants are usually checked before trials too. Resistance to vaccination begins early at age 30 and. COVID vaccines are being rolled out across the UK and potentially another vaccine could soon be approved for use.
If vaccination continues at. The approved vaccines require two doses to provide the best protection against Covid. Are there any side effects to a Covid vaccine.
How many coronavirus vaccines are there. No one knows who has the vaccine until someone is infected with Covid-19 and the reaction is studied. The NHS vaccination programme aims to immunise about 14 million people at greatest risk of Covid by mid-February with second doses to be given up to 12 weeks later.
In England the vaccine is being offered in some hospitals and pharmacies at local vaccination centres run by GPs and at larger vaccination centres. The data also showed a strong immune. Once vaccines are demonstrated to be safe and efficacious they must be approved by national regulators manufactured to exacting standards and distributed.
The latest update on COVID-19 vaccinations was released on 31st December 2020 and can be found here. The UK became the first country in the world to approve the PfizerBioNTech coronavirus vaccine. There is evidence the Oxford vaccine can also reduce the spread of the virus.
The Moderna vaccine was approved on 7 January while Pfizer vaccine the first to be rolled out was approved on 2 December 2021. The MHRA authorisation includes conditions that the AstraZeneca Oxford vaccine should be administered in 2 doses with the second dose given between 4 and 12 weeks after the first. Some believe mRNA vaccines are safer for the patient as they do.
They both require 2 doses to provide longer-lasting. WHO is working with partners around the world to help coordinate key steps in this process including to facilitate equitable access to safe and effective. It was approved late in 2020 after trials showed that it stopped 70 of people developing Covid symptoms.
In the UK there are 2 approved COVID-19 vaccines. The roll-out of the Oxford vaccine began on 5 January. An effective COVID-19 vaccine also faces several hurdles beyond our control.
Two of the COVID-19 vaccines now approved for use in the UK by the MHRA are in the process of being rolled out the Oxford-AstraZeneca vaccine and the Pfizer-BioNTech vaccine. Instead we see there is a much larger number of people who get Covid-19 in the group who did not get the vaccine. She noted that most of.
A third the Moderna vaccine has been approved and will begin to be given in Spring. This isnt actually a quick sprint this is a marathon. Who can get the COVID-19 vaccine The NHS is currently offering the COVID-19 vaccine to people most at risk from coronavirus.
People most at risk from the complications of COVID-19 are being offered the vaccine first. In trials for the PfizerBioNTech vaccine only 94 of 43538 participants contracted Covid-19. More than 7 million people have now received their first dose of a vaccine with over 40000 receiving a second dose.
The older we get the poorer our ability to respond to vaccines.

Why The Covid 19 Vaccine Needs To Be Kept So Cold Houston Methodist On Health

Uk Covid Vaccine Rollout Begins With Vaccination Of 90 Year Old Woman

How Much Will A Covid 19 Vaccine Cost Financial Times
Covid Vaccine Uae 250 000 Residents Get Second Dose News Khaleej Times

Covid Vaccine Fda May Authorize Pfizer S This Week
Covid Vaccine Cdc Should Warn People The Side Effects From Shots Won T Be Walk In The Park

Covax Global Covid Vaccine Program Secures Nearly 2 Billion Doses For Unicef Distribution

Covid Vaccine Uk What Vaccines Are There When Will They Be Ready Bbc Science Focus Magazine
The World Needs A Covid Vaccine Will There Be Enough For Everyone Barron S

Pfizer Vaccine In Uae Dubai Abu Dhabi More Registration Where To Get Price Etc Updated 18 January 2021 Wego Travel Blog

Covid 19 Risks And Side Effects Of Vaccination Science In Depth Reporting On Science And Technology Dw 20 01 2021
Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center

Fda Approves Covid Vaccine How And When Will I Get It

Not Enough Covid Vaccine For All Until 2024 Says Biggest Producer Financial Times
Covid Vaccine You Can T Sue Pfizer Or Moderna Over Side Effects
Covid Vaccine U S Plans To Ship 6 Million Moderna Doses Once Fda Gives Ok
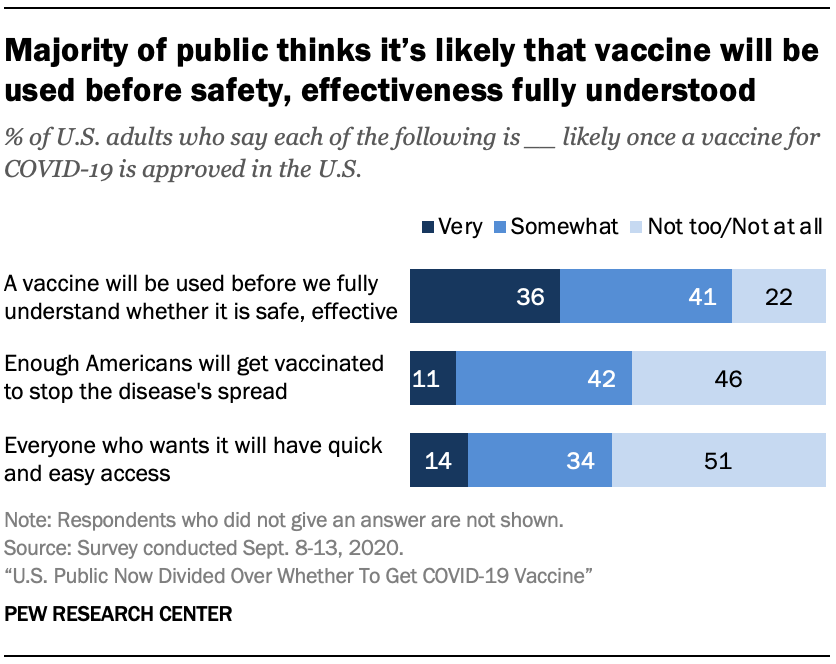
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center

