Infliximab Antibodies
antibodies infliximab wallpaperTreatment with infliximab can result in the formation of antibodies against infliximab. Presence of infliximab in serum up to 100 ugmL and all.
INFLIXIMAB ANTIBODY Patients receiving Infliximab drug experience loss of response to therapy during course of disease primarily due to development of antibodies to Infliximab.

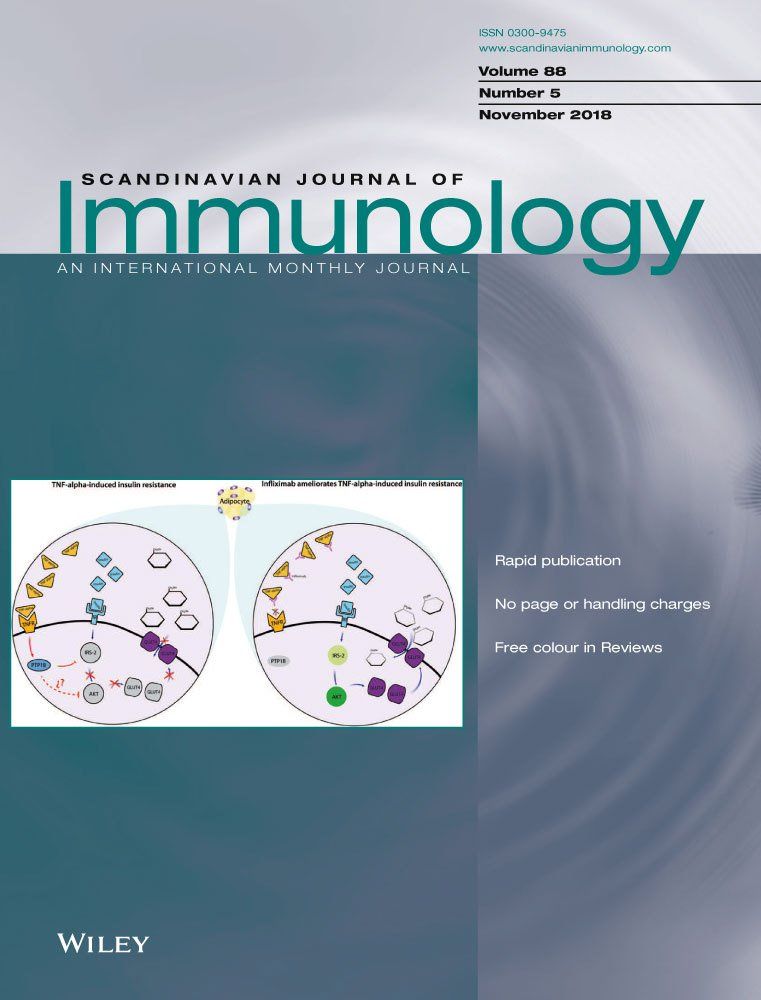
Infliximab antibodies. Anti-drug-antibodies ADA against infliximab are frequently measured in patients receiving infliximab treatment with loss of response and undetectable infliximab concentrations. Using these ADA panels we compared the detectability of model methods ie binding assays using SPR BLI and ECL and a cell-based assay to detect neutralization activity. Infliximab Remicade is a chimeric mouse-human antibody directed against human tumor necrosis factor TNF.
Infliximab Level and Anti-drug Antibody for IBD - When treatment of inflammatory bowel disease with infliximab or its biosimilar fails a physician may need to consider treatment options such as adjusting dose or dosing intervals switching to a different TNF blocker or switching to a non-TNF blocker. Infliximab Anti-drug Antibody for Rheumatic Diseases - When treatment of rheumatic diseases with infliximab or its biosimilar fails a physician may need to consider treatment options such as adjusting dose or dosing intervals switching to a different TNF blocker or switching to a different drug agent ie. In the presence of anti-infliximab antibodies the infliximab drug level typically reflects the antibody-unbound fraction of infliximab concentration in serum.
The biosimilar CT-P13 branded as Inflectra Hospira and Remsima Celltrion is approved in Europe for use across all indications of Remicade. The developed ADA panels consist of 7 for infliximab and 11 for adalimumab ADAs with various binding characters and most of the ADAs are neutralizing antibodies. The presence of these antibodies has been associated with infusion reactions in 7-19of patients and may also shorten the duration of the effect of infliximab when given repeatedly.
Infliximab a monoclonal antibody that works against tumor necrosis factor alpha is marketed as Remicade Janssen Biotech. Infliximab is a. Inflixmab is FDA-approved for the treatment of rheumatoid arthritis and Crohns disease.
Infliximab a chimeric monoclonal antibody sold under the brand name Remicade among others is a medication used to treat a number of autoimmune diseases. Infliximab branded as Remicade is a chimeric monoclonal antibody drug IgG1kappa that has been approved for treatment of psoriasis Crohns disease ankylosing spondylitis psoriatic arthritis rheumatoid arthritis and ulcerative colitis. The Quantum Blue Anti-Infliximab rapid test allows for a highly specific detection of antibodies against the biologic within 15 min.
In one small study comparing three popular biologic medications it was found that anti-drug antibodies were present in 42 of those receiving Remicade infliximab 33 of those receiving Humira adalimumab and in none of those patients receiving Enbrel etanercept. They are ideal for development of a pharmacokinetic PK bridging ELISA to measure free drug. Remicade belongs to a drug class called tumor necrosis factor-alpha TNF-alpha blockers.
This test 1 measures infliximab and infliximab-dyyb inflectra levels to help differentiate pharmacodynamic or pharmacokinetic conditions to guide therapy and 2 tests for anti-drug. My notes and questions. Positive antibody results are verified by a confirmatory.
Remicade contains the drug infliximab which is a biologic a drug made from parts of living organisms. The presence of anti-drug antibodies ADA against infliximab can diminish the effect of the biologic drug infliximab. Infliximab and adalimumab are therapeutic monoclonal antibodies that target tumor necrosis factor-alpha TNF-a a specific proinflammatory molecule.
Anti-Infliximab Inhibitory Antibodies Type 1 Type 1 anti-infliximab antibodies inhibit the binding of the drug infliximab to its target tumor necrosis factor alpha TNFα. This includes Crohns disease ulcerative colitis rheumatoid arthritis ankylosing spondylitis psoriasis psoriatic arthritis and Behçets disease. The presence of infliximab drug even at concentrations well above target treatment levels 50 μgmL does not interfere with the anti-infliximab antibody detection.
In fully human IgG1 format they are suitable as a surrogate positive control or calibrator for an anti-drug antibody ADA assay. It is given by slow injection into a vein typically at six- to eight-week intervals. They are mostly prescribed to treat rheumatic diseases inflammatory bowel disease and certain dermatologic conditions.
The presence of infliximab drug even at concentrations well above target treatment levels 50 ugmL does not interfere with the anti-infliximab antibody detection. In the absence of anti-infliximab antibodies the infliximab drug level typically reflects the total infliximab concentration in serum. In the presence of anti-infliximab antibodies the infliximab drug level typically reflects the antibody-unbound fraction of infliximab concentration in serum.
These antibodies bind to the drug and either block their action or increase their clearance leading to loss of clinical efficacy. Common side effects include infections acute infusion reactions and abdominal pain. Food and Drug Administration FDA has CT-P13 under consideration.
Quantum Blue Anti-Infliximab. Infliximab Remicade Renflexis Inflectra is a chimeric immunoglobulin IgG1 kappa targeting tumor necrosis factor-alpha TNF-a and it is currently FDA-approved for the treatment of multiple inflammatory conditions. I was only on 3 vials of Remi I should have been on 4 for my weight but they never bothered to weigh me at the time this bloodwork was taken.